___ Described the Location of Electrons Using 4 Quantum Numbers
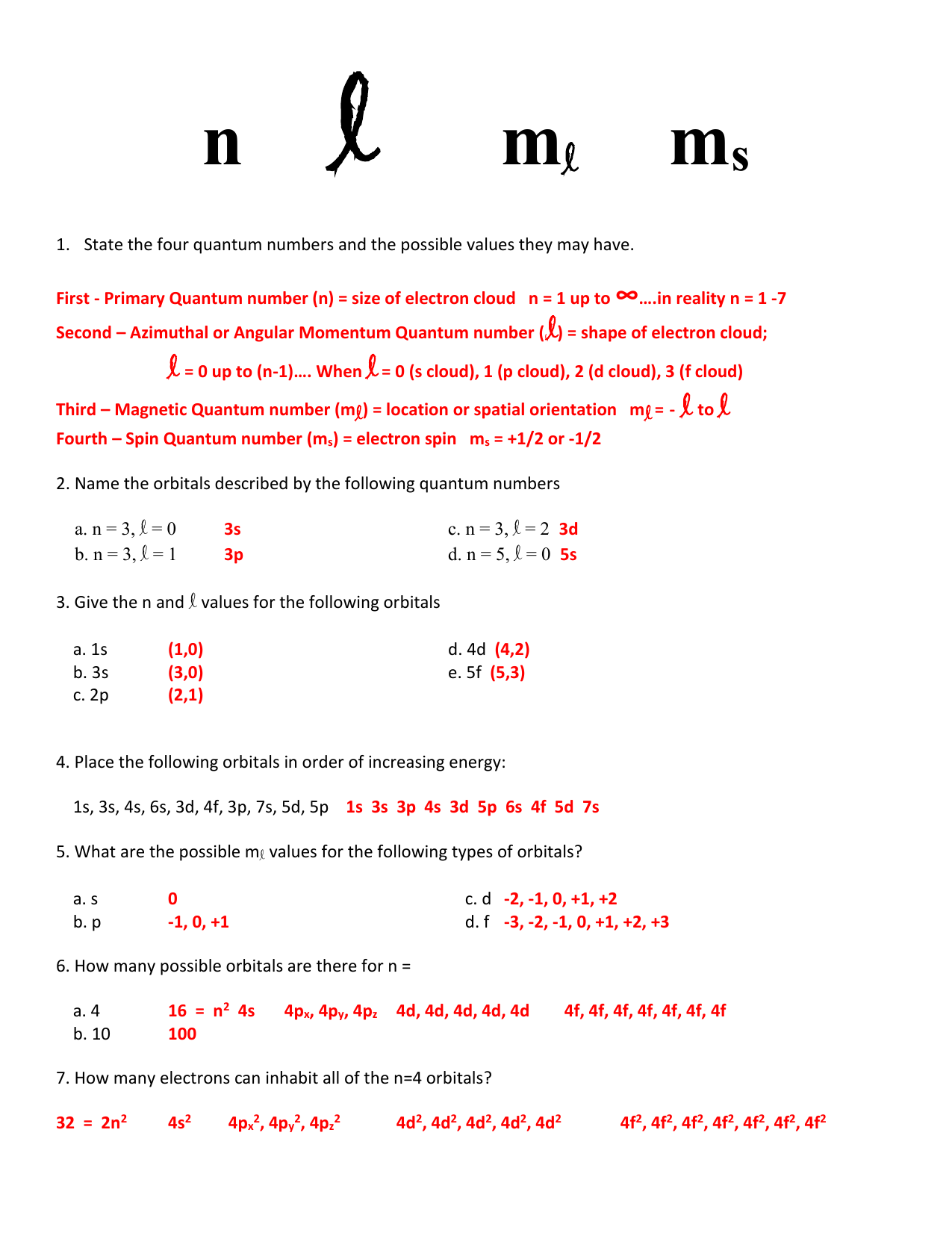
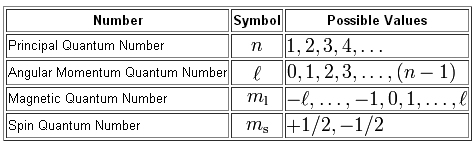
An electron in an atom or ion has four quantum numbers to describe its state are principal quantum number azimuthal quantum number magnetic. More specifically the four quantum numbers will tell you the energy level on which the electron resides - given by n the principal quantum number the subshell in which the electron resides - given by l the angular momentum quantum number the exact orbital in which you can find the electron - given by m_l the magnetic quantum number the spin of the electron - given.
1 5 1 Quantum Numbers Worksheet Key
The carbon C has 6 electrons it s electronic configuration is.

. As you know four quantum numbers are used to describe the location and spin of an electron in an atom. The number and letter pairs in an electron configuration represent two of the electrons four quantum numbers. 100 1 rating Transcribed image text.
These quantum numbers tell us more information about the properties of electrons and. The first quantum number describes the electron shell or energy level of an atom. The four quantum numbers used to describe the electrons are n2 ℓ1 m1 0 or -1 and s12 the electrons have parallel spins.
This video shows you how to identify or determine the 4 quantum numbers n l ml and ms from an element or valence. N 72 111 et 7 elections 112 ins 2. This video provides 3 example practic.
For a calcium 2 ion there will be 18 sets of quantum numbers not the usual 20 for a neutral atom. Quantum numbers a series of specific numbers to describe the location of an electron of a particular atom. The location or address of the electrons is given by four numbers called quantum numbers.
Quantum Numbers Write the four quantum numbers which describe the location of the highest energy electron of the following. It will be missing the two electrons in the 4s sublevel. The value of n ranges from 1 to the shell containing the outermost electron of that atom.
กร ne M2 Ms Conceptual Exercise 9. Considering this what do the four quantum numbers describe about an electron answers com. Energy n angular momentum ℓ magnetic moment m ℓ and spin m s.
True As the value of n increases the average distance of the electron cloud from the nucleus increases and the energy of the electron in that shell also increases. Which tells you the subshell in which you can find the electron. So lets start with the first one the principal quantum number which tells you the energy level on which an electron resides.
Principle Quantum Number n. What is the principal quantum number of an electron. Which of the following sets of quantum numbers can describe a 4s electron.
According to the Pauli Exclusion Principle each electron in an atom has an exclusive set of quantum numbers and no two electrons can have the same combination of four quantum. These numbers will refers only to the elements highest energy electron because the other fall into the same locations that have been described in the elements preceding it. So the fourth electron is in the 2s orbital the four quantum to describe it would be.
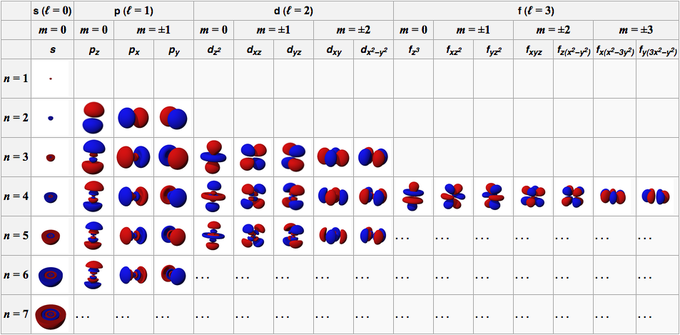
This tells us that there are two electrons per orbital or per ml value one with an ms value of 1 2 and one with an ms value of 1 2. These quantum numbers refer to different locations on the periodic table and different orientations of. Therefore n describes the shell both n and l describe a subshell n l and ml describe an orbital and all four quantum numbers n l ml ms describe an electron.
This is our final way to describe the location of an electron. Two electrons in the same orbital may be identified with a spin quantum number. To completely describe an electron in an atom four quantum numbers are needed.
Ni 28 -12 28 electron 16 e o mi ms 3. Each electron in an atom has a set of four quantum numbers which uniquely describe the location of that electron in terms of energy. A n 4 ℓ 0 mℓ 0 B n 3 ℓ 0 mℓ 0 C n 3 ℓ 2 mℓ 0 D n 4 ℓ 1 mℓ 0 E n 3 ℓ 1 mℓ 0.
Learn All electrons have four quantum numbers which describe the location of electrons in the electron cloud of an atom and can be used to determine the electron configuration of an atom. It consists of four numbers that act as coordinates to locate the electrons position. Quantum numbers define the features of the atomic orbitals and the electrons filled in those orbitals.
Quantum Numbers Introduction To Chemistry

Quantum Numbers For The First Four Shells Video Khan Academy

Homework 10 Worksheet Quantum Numbers Ppt Download
Quantum Numbers Introduction To Chemistry

Quantum Numbers Video Quantum Physics Khan Academy

1 Fill In The Blanks A The Principal Quantum Number N Can Have Integer Values From B Homeworklib



Comments
Post a Comment